Compiled by Professor Dr Md. Mozammel Hoq
An immunology expert describes various tests for coronavirus, whether we’re immune to COVID-19 after infection, and more.
ABOUT
Such antibody testing has already started, but it can’t ramp up overnight. And first, scientists need to figure out exactly what to test for, and whether having these antibodies actually make someone immune and for how long, says Yvonne Maldonado, MD, a professor at the Stanford University School of Medicine.
“Right now, we’re trying to do some studies to understand exactly what having antibodies really means,” she says.
A virus-like the new coronavirus, officially called SARS-CoV2, enters cells and hijacks their machinery to make more copies of itself. The immune system then makes antibodies to track down and kill these clones, says Aneesh Mehta, MD, an infectious disease specialist at Emory University School of Medicine in Atlanta.
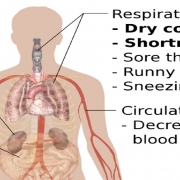
While diagnostic tests can tell if someone is currently infected, testing for antibodies reveals whether they’ve ever been infected — even if they never felt sick.
Experts say these antibody tests, in the short term, can answer personal questions, like, “Was I infected?” It will take much longer to answer questions such as, “How long will immunity last after infection?” and societal ones, such as, “How dangerous is COVID-19 really?”
And until we know the answer to those questions, we won’t really know the true value of having antibodies. While experts agree it doesn’t mean our lives will completely revert to the way they were before, the tests can help us get on that path.
Knowing how many people were actually exposed and developed antibodies will help officials understand how dangerous it truly is, says Michael Mina, MD, PhD, an assistant professor of epidemiology at the Harvard T.H. Chan School of Public Health. While millions of people worldwide have been diagnosed with COVID-19, many more have probably had it and were unable to get tested or didn’t even notice the infection.
“It really changes our view of many, many things: First and foremost, how many people have been infected and [how many] remain susceptible to this infection,” he says. “But it also changes our view on the actual biology and pathogenicity of this virus. It will determine and change how we’re looking at this virus.”
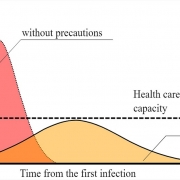
Antibody tests will also be essential for getting us out of our houses and back to work, and for easing the fear that has paralyzed the country, says Marc Lipsitch, PhD, also an infectious disease epidemiologist at Harvard.
Policymakers will need to know how many people have the disease and how many have immunity against it, Lipsitch says, before deciding when it’s safe to loosen social distancing requirements and when they will need to tighten up again to cope with a new wave of infections.
Hopefully, having antibodies will protect someone from getting COVID-19 a second time. But since the virus has only been around since late last year, no one yet knows how long that protection will last.
With the common cold, a relative of the new coronavirus, immunity doesn’t last long. You can catch it again a few months later. On the other hand, people who contracted severe acute respiratory syndrome (SARS) — another related virus that caused a deadly outbreak in 2003-2004 — still carry protective antibodies more than 15 years later, Maldonado says.
Where this virus lies on that continuum could have a huge impact on the country’s ability to reopen and get people back to work, and also on the development of a vaccine, experts say.
If immunity lasts for years, those who have recovered can generally relax, resume their daily lives, and go back to hugging loved ones.
If immunity lasts for just a short time, then even people who were infected once could be vulnerable again soon — and it will be harder to develop a protective vaccine, says Matthew Sims, MD, PhD, director of infectious disease research at Beaumont Health.
Ramping Up Testing
Researchers are also still deciding which antibodies their tests should look for. Some antibodies are made early in an infection and go away, usually within a few weeks, while others can linger for months or years. Looking for antibodies called Immunoglobulin M, or IgM, can identify recent infections, says Harvard’s Mina.
Immunoglobulin G, or IgG, stays around longer, he says. “I would choose IgG for that effort to get a better understanding of how many people have gotten it,” he says, noting that tests for IgG usually yield information about IgM as well. So far, all of the announced tests look for IgG.
A third antibody, Immunoglobulin A, or IgA, plays a role in the immune function of mucous membranes, Sims says, and will be part of the test he is launching.
ABOUT
There are also different types of tests, Sims says. The commercial tests often use a finger-prick of blood and reveal a “yes/no” answer, like a pregnancy test. Cellex’s test, which takes about 15-20 minutes to yield results, is one of only three that the FDA has approved so far.
Abbott’s test, which is being rolled out on April 16, can be analyzed on any of 2,000 machines that are already in labs across the United States, says a company spokeswoman. Each machine can run 100-200 tests a day at a cost of about $6 each.
Sims says most of these commercial tests just say whether the person has ever been infected, while most of the tests used for research also look for the amounts of antibodies.
The amount would be useful for a few reasons, including finding out if a person’s blood is suitable for donating convalescent plasma, a blood product from someone who has recovered from COVID-19 that can be used to treat people who are fighting the infection and that has antibodies against the virus. For instance, Harvard President Lawrence Bacow, who recently recovered from a COVID-19 infection, donated blood this week in hopes of helping someone who is still fighting it off. In Kentucky, people who are tested for antibodies will be told if they have enough to become donors.
Antibody tests also vary in their reliability, sometimes returning false positives — identifying someone as having had the disease when they haven’t — and false negatives — missing people who have antibodies in their blood.
Maldonado says the test her team has developed at Stanford is reasonably reliable, correctly identifying blood samples with antibodies to the virus the vast majority of the time. Some commercial tests, she says, are not as specific and might find antibodies to the viruses that cause the common cold, for instance, which are similar to the new coronavirus.
Source: WebMD Health News, April 16, 2020