COVID-19 Vaccine
(Compiled by Dr. Md. Mozammel Hoq)
As the new coronavirus continues to spread, people around the world are anxious to know when we might have a vaccine to stop it.
What Would a COVID-19 Vaccine Do?
When you come into contact with a virus or bacteria, your body’s immune system makes antibodies to fight them off.
A vaccine forces your immune system to make antibodies against a specific disease, usually with a dead or weakened form of the germs. Then, if you come into contact with them again, your immune system knows what to do. The vaccine gives you immunity, so you don’t get sick or so your illness is much milder than it otherwise would have been.
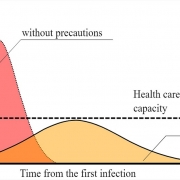
A vaccine against COVID-19 would slow its spread around the world. Fewer people would get sick, and more lives could be saved.
How Are Vaccines Developed?
So how long could a COVID-19 vaccine take? Dozens of possible vaccines are in various stages of development around the world, according to the World Health Organization. Some have begun clinical trials. But certain things can’t be rushed, like how long it takes a person’s immune system to respond to a vaccine or the wait to check for side effects.
Even when researchers find a vaccine that works against the new coronavirus, it could be 12 to 18 months at best before it’s ready for the public. That’s only a fraction of the usual time.
Before any vaccine can be used widely, it must go through development and testing to make sure that it’s effective against the virus or bacteria and that it doesn’t cause other problems. The stages of development generally follow this timeline:
- Exploratory stage. This is the start of lab research to find something that can treat or prevent a disease. It often lasts 2 to 4 years.
- Pre-clinical stage. Scientists use lab tests and testing in animals, such as mice or monkeys, to learn whether a vaccine might work. This stage usually lasts 1 to 2 years. Many potential vaccines don’t make it past this point. But if the tests are successful and the FDA signs off, it’s on to clinical testing.
- Clinical development. This is a three-phase process of testing in humans. Phase I usually lasts 1 to 2 years and involves fewer than 100 people. Phase II takes at least 2 years and includes several hundred people. Phase III lasts 3 or 4 years and involves thousands of people. Overall, the clinical trial process may stretch to 15 years or more. About a third of vaccines make it from phase I to final approval.
- Regulatory review and approval. Scientists with the FDA and CDC go over the data from the clinical trials and sign off.
- Manufacturing. The vaccine goes into production. The FDA inspects the factory and approves drug labels.
- Quality control. Scientists and government agencies keep tabs on the drug-making process and on people who get the vaccine. They want to make sure it keeps working safely.
This version of the coronavirus only surfaced in late 2019, but scientists have gotten a boost from research on similar coronaviruses that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). Efforts to fight those diseases played a large role in the record speed of the first COVID-19 vaccine trial that’s now underway.
Some of the companies working on vaccines are also looking for ways to ramp up production quickly when the clinical trials find one that works safely. With more than 300 million people in the United States alone, mass vaccination will be a joint effort among several companies and government agencies.
Experts say the coronavirus could turn out to be seasonal, like colds and the flu. A vaccine might not be ready until after the current pandemic is over, but it may be vital if the cycle begins again.
(WebMD Medical Reference Reviewed on April 03, 2020)







Leave a Reply
Want to join the discussion?Feel free to contribute!